| Blood plasma | [German version] |
Table of contents |
|
| General: | ||
| Product information | ||
| Packaging | ||
| Transport | ||
| Container transport | ||
| Cargo securing | ||
Product information
Product name
| German | Blutplasma |
| English | Blood plasma |
| French | Plasma Sanguin |
| Spanish | Plasma Sanguíneo |
| Scientific | Plasma Sanguinis |
| CN/HS number * | 3002 10 95 |
(* EU Combined Nomenclature/Harmonized System)
Product description
Blood plasma is the liquid component of blood that holds the cellular components of blood in suspension. These include erythrocytes (red blood cells), leukocytes (white blood cells) and thrombocytes (platelets) [1]. It is a slightly cloudy liquid whose color varies from pale yellow through bright green from person to person.[2] It makes up about 55 % of the total blood volume, as determined by centrifuging.[3] Blood plasma that does not contain clotting factors is referred to as blood serum.[4]
Blood plasma is 90 % water. The remaining 10 % is made up of substances dissolved in water: Plasma proteins, electrolytes and low-molecular substances such as vitamins, trace elements, hormones and nitrogenous excretory products. One of the most important tasks of blood plasma is to act as a medium for the transportation of cellular components.[5]
Of particular importance are the plasma proteins, of which approximately 100-120 have been identified to date. The proteins include the albumins, the clotting factors, the inhibitors and the immunoglobulins (also known as antibodies).[6]
Blood preparations, including blood plasma, are pharmaceutical products as defined by Section 4, Paragraph 2 of the German Pharmaceutical Products Act (AMG). They are classified as transfusion products and plasma derivatives. Because these preparations contain biological substances, they are classed as biomedical products.[7]
Blood plasma is obtained by centrifuging whole blood donations or by plasma apheresis.[8] All donated blood is screened for pathogens (e.g. HIV, hepatitis). Only plasma that has tested negative is permitted to be used or processed![9] Blood plasma is quarantined for at least 4 months (transfusion plasma)[10] or 60 days (industrial plasma)[11] before use.
Blood plasma can be prepared in three different ways:
| Type | Features | Source material | Use |
| Frozen fresh plasma (FFP)[12] | Frozen at approximately -30 °C; ready for use after thawing in a quality-controlled process; when thawed, it is a clear liquid with no solid substances | Plasma from a single donor | Transfusion or industrial processing |
| Lyophilized human plasma (LHP)[13] | Freeze-dried, white or yellowish powdery substance; ready for use for injection purposes after a few minutes when dissolved in water; cell-free | Plasma from a single donor | Transfusion |
| Virus-inactivated plasma (SDP – solvent-detergent plasma)[14] | Virus-inactivated with a special process (typically solvent-detergent); cell-free; approximately 10-15 % lower clotting factor activity than with FFP or LHP; quarantine not necessary; supplied frozen or freeze-dried | Plasma pool (plasma from 500 – 1600 individual donors) | Transfusion or industrial processing |
After the plasma has been thawed or dissolved, it must be used within 6 hours in order to prevent contamination.[15] The manufacturer's instructions with respect to correct use, transportation and storage must always be observed.
The Paul Ehrlich Institute (PEI) in Langen, is the supreme federal authority responsible for blood products in Germany. According to the PEI listing, blood plasma falls into the category "Blood Components for Transfusion" alongside thrombocyte and erythrocyte concentrates. Blood plasma preparations must be approved by the Paul Ehrlich Institute in Langen. Approval notices are published regularly in the German Federal Gazette.
Quality/Duration of storage
After it has been thawed or dissolved in water for the purpose of injection, blood plasma must not contain solid or gelatinous substances.[16]
Plasma is not a sterile product. All plasma units contain a small quantity of microorganisms and pathogens. However, because blood plasma is stored in hermetically sealed containers, sterile storage is not necessary.
One of the aspects affecting the quality of the blood plasma is the activity of the plasma proteins it contains. Excessive temperatures during transport and storage cause this activity to decrease and thus degrade the quality of the final product.
The maximum storage duration of industrial plasma is 36 months as of the time of donation. Transfusion plasma, on the other hand, may not be used beyond 24 months after the time of donation.[17]
Intended use
There are two major fields of application: transfusion products, which are used directly in transfusion medicine, and plasma derivatives. These are essential, highly effective medicinal preparations manufactured from the 100-plus plasma proteins by means of fractionation[18]:
| Transfusion plasma applications[19] | Plasma derivative applications[20] |
| Significant loss of blood | Factor concentrates |
| Significant invasive intervention | Inhibitor concentrates |
| Clotting factor V or XI deficiency | Fibrin glue |
| Volume replacement fluids | Drugs for immune disorders |
| Vaccines | |
| Anti-D immunoglobulins |
The clotting factors V and XI cannot be manufactured as concentrates and are therefore not available as plasma derivatives.[21]
Transfusions of blood plasma must always be ABO-compatible. In cases of emergency, where it may not be possible to test the blood group of the recipient, AB group plasma can be used for transfusion, because this is compatible with all other blood groups.[22]
Countries of origin
Blood plasma is produced throughout the world. If plasma is imported into the European Union to be used as a raw material for manufacturing drugs, the member states of the EU must ensure that the plasma is only imported if it is subject to comparable regulations in the country of origin and if the EU directives are observed during transport and in respect of storage and documentation.[23]
Plasma is imported into the EU primarily from the USA.
Back to beginning
Packaging
Both plastic and glass can be used as primary packaging for blood plasma, with glass containers only being used for lyophilized plasma.
Plastic containers are generally made of polyethylene, polypropylene and plasticized PVC. Permitted additives include antioxidants, plasticizers, stabilizers and lubricants.[24] The plastic containers must be capable of withstanding extremely low temperatures.[25] Plastic bags come in sizes designed for storing between 250 ml and 750 ml. The plastic bottles generally contain between 650 ml and 850 ml, depending on the volume donated, but it is also possible for them to contain less.
Glass containers are made from soda-lime glass with a high hydrolitic resistance. The interior and exterior surfaces of the glass may be treated to optimize its chemical and physical properties. Glass containers must not be reused. The glass bottles generally contain 200 ml of lyophilized plasma, which is dissolved in 200 ml of water for injection purposes.[26]
Because the primary packaging is in direct contact with the blood plasma, the packaging material used must give off no substances to the blood plasma that can in any way impact on its purity or effectiveness or that represent a toxicity risk. The containers must be transparent to permit visual inspection.[27] It must be possible to hermetically seal the containers and it must be possible to open or dismantle them easily.[28]
A traceability system is obligatory. This system must use an exact identification process, a specific labeling system and precisely defined documentation processes in order to absolutely guarantee that each and every blood preparation can be reliably assigned to both the donor and to all recipients.[29] Suitable labeling with no chance of errors is vital in achieving this. In Germany, the stipulations laid down in Section 10, "Labeling", of the German Pharmaceutical Products Act (AMG) apply. These stipulations state that, in addition to the product designation, the expiry date and the storage temperature must be declared on the plasma packaging. Within the EU, the international labeling system ISBT 128 (ICCBBA standard) is also used.[30] The figure below shows an example of such a label. Although the figure shows a label for blood platelets, the important thing is the structure and arrangement of the label, which apply equally to labels for blood plasma.
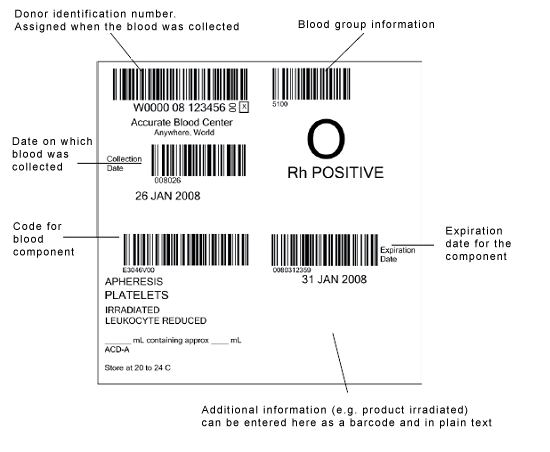
Figure 1: International ISBT 128 blood component label as specified in the ICCBBA standard. www.iccbba.org, (source: Optimal Blood use, EU,
http://www.optimalblooduse.eu/content/62-blood-component-label, 20 March 2012)
As a minimum, the following details must be provided on the labels:
- The official designation of the blood component
- The volume and weight of the blood product
- A uniform numeric/alphanumeric identifier for the donation
- The name and address of the blood donation organization or pharmaceuticals company that manufactured the product
- The ABO group (transfusion plasma only)
- Any expiry date
- The required storage temperature
- If applicable, the virus inactivation method used
- The necessary details on the anticoagulant used.[31]
All data that permits comprehensive traceability must be retained for at least thirty years and must be able to be accessed immediately.[33]
There are no official regulations governing the secondary packaging and transport packaging. Consequently, general requirements such as low weight, simple manual or mechanical handling and optimum use of transport and storage space apply. Furthermore, it should be fit for purpose in order to ensure that it is able to withstand the loads experienced during transportation.[34]
Back to the beginning
Transport
Symbols
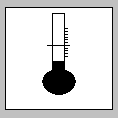 Temperature-controlled |
Means of transport
Ship, truck, aircraft
Container transport
Refrigerated container
Legal requirements
When transporting and storing blood plasma, the "Good Manufacturing Practices" (GMP) and "Good Distribution Practices" (GDP) must be observed in conjunction with the German Pharmaceutical Products Act (AMG). These stipulate standard operating procedures (SOPs). Standard operating procedures are drawn up by each manufacturer and will normally form part of the framework agreements with the shipping agents and carriers. Cleanliness of the means of transport and warehouses is an essential requirement.
If any deviations from the standard operating procedures are identified or if any damage is discovered during transportation, this must be analyzed and assessed by the manufacturer's quality department. The quality department then decides whether any damage has occurred or whether the deviation is within acceptable tolerances.
In some countries, the companies and means of transport used for the transportation of blood plasma may be regulated in accordance with legislation pertaining to pharmaceutical products and may therefore need to provide approval documentation or to have completed examinations as laid down in national law.
The storage conditions laid down by the manufacturer must be observed throughout the entire transport chain for pharmaceutical products.
Cargo handling
In order to avoid interruption of the cold chain as far as possible, the product should be transported directly wherever this is feasible. The specified temperatures must always be observed during cargo handling. In order to protect staff and the blood plasma, the packaging that has been used must not be damaged. Glass containers and plastic containers are sensitive when frozen. And cargo handling should therefore be carried out as carefully as possible.
Stowage factor
The stowage factor and weight vary considerably because different containers containing different volumes are used.
Stowage space requirements
Cool, dry, air circulation necessary
The particular demands of the refrigeration units and means of transport used must be taken into account in order to ensure refrigeration during transportation. There must be adequate air circulation between the refrigeration unit and the warm air in the hold of the means of transport in order to make sure that the units operate efficiently. This means that the air must be able to flow between the side walls or container walls and around the cargo. If there are no corrugations or similar in the loading bed, the cargo should be placed on pallets. The side walls are often marked to indicate the maximum loading height. These should not be exceeded in order to ensure good air circulation.
Separation
Blood plasma must not be transported and stored in the same container as foodstuffs, other pharmaceutical products or other materials.[35]
Cargo securing
To prevent the transport units from slipping or tilting, the cargo should be adequately bundled to form cargo units. The cargo units must also be secured properly in the means of transport.
Back to beginning
Risk factors and loss prevention:
RF Temperature
Blood plasma must be deep frozen as soon as possible after centrifuging or after it has been obtained by means of plasma apheresis. Ideally, this should be done within the first six hours. Statutory regulations prescribed a maximum of 24 hours. Regulations require a freezing method that guarantees complete freezing to at least -30 °C within one hour. It is critical that the temperature falls below the eutectic point, which is -23 degrees Celsius for plasma.[36]
Before the plasma is stowed in the hold/container, the current internal temperature of the hold/container must be checked. The cargo should only be stowed if the temperature is correct. Holds/containers should therefore be precooled prior to loading.
| Designation | Temperature |
| Frozen fresh plasma (FFP) Statutory requirement[37] Recommendation on the basis of the eutectic point[38] |
< -20 °C < -27 °C |
| Lyophilicized human plasma (LHP)[39] | +2 °C through +25 °C |
The holds/containers must be equipped with measuring equipment that permits continuous measurement and storage of measurements to allow constant recording and documentation of the temperature. Specified temperatures should be recorded on the transport document. The carrier must inform the recipient of the blood plasma about any deviation from the defined temperature. In addition, the recipient should check the records for any discrepancies when the goods are inspected at each interface.[40]
Data loggers should be used to record the temperatures within the cargo unit independently of the means of transport. The positions of the measuring points and the number of data loggers to be used is generally prescribed by the manufacturer, otherwise it should be determined by an independent expert. Multiple data loggers should be used within each cargo unit in order to ensure accurate data. The positions of the data loggers should be recorded in the transport document to facilitate subsequent checking of the measurements.
The FFP units should never be allowed to thaw, either partially or completely. The European Pharmacopoeia allows for one exception. The temperature may exceed the specified -20 °C for a maximum of 72 consecutive hours, provided that it remains below -15 °C. But this threshold may also be exceeded once within the 72 hours, provided that the temperature is not above -5 °C.[41]
Transportation or storage at excessive temperatures causes the protein concentration to fall and may result in an increase in microorganisms and pathogens. If safety concerns prevent the plasma from being processed or transfused, it must be discarded.
Back to beginning
RF Humidity / Moisture
Maximum equilibrium moisture content 70 % (relative to the paperboard of the cartons)
The particular humidity condition guidelines relate to the packaging material and packaging aids used. If they are hygroscopic, their shape or strength changes under the influence of moisture or humidity. Single-wall corrugated board cartons, for instance, lose more than 65% of their edgewise crush resistance under conditions of high humidity or in the case of moisture penetration. This may necessitate repackaging the blood plasma in different transport packaging to ensure that it remains capable of being stacked and handled.
The surface of the blood plasma packaging begins to "sweat" if the dew point temperature of the external air is lower than the temperature at the surface of the packaging. This condensation water can also have a negative impact on the secondary packaging.
Back to beginning
RF Ventilation
This risk factor has no major influence on transportation of this product.
Back to beginning
RF Biotic activity
This risk factor has no major influence on transportation of this product.
Back to beginning
RF Gases
This risk factor has no major influence on transportation of this product.
Back to beginning
RF Self-heating / Spontaneous combustion
This risk factor has no major influence on transportation of this product.
Back to beginning
RF Odor
This risk factor has no major influence on transportation of this product.
Back to beginning
RF Contamination
| Active behavior | Frozen blood plasma does not cause contamination. |
| Passive behavior | Blood plasma is extremely sensitive to contamination. However, because the primary packaging must be hermetically sealed, contamination can only arise when the containers are opened (during transfusion or fractioning). |
Back to beginning
RF Mechanical influences
Frozen blood plasma is largely insensitive to mechanical influences.
LHP is principally transported and stored in glass containers, which are highly sensitive to impact. Damage to the glass containers can lead to contamination of the plasma. The transport packaging should therefore be designed in such a way that it is able to absorb the loads experienced when it suffers impact or is dropped. The packaged goods should also be protected from knocking against each other.
Back to beginning
RF Toxicity / Hazards to health
The welded edges of the plasma bags and the welded tubes of the plasma bottles are extremely sharp when frozen. These represent a risk of serious injury to staff handling the individual packs.
In the case of blood plasma that has not yet been screened for pathogens and been declared as negative, there is always a risk that it may contain life-threatening pathogens such as HIV or hepatitis. For this reason, those involved in obtaining the plasma, in particular, should wear gloves and avoid any direct contact with the blood or blood plasma of the donor. If the plasma is frozen or freeze-dried, the risk of infection is reduced to a minimum, but cannot be entirely eliminated.
If any leaks are detected in bottles or bags, they should immediately be discarded in order to protect the recipient and the staff. Furthermore, no attempt should be made to open plasma bottles and bags during transportation or storage because of the risk of infection (e.g. with HIV or hepatitis).
Back to beginning
RF Shrinkage / Shortage
The traceability system means that there is no market for stolen blood plasma in Europe.
Back to beginning
RF Insect infestation / Diseases
Thawed or dissolved blood plasma must be transfused or processed within six hours, otherwise the microorganisms and pathogens contained in the plasma multiply rapidly and contaminate the blood plasma. If the plasma is not used within this period, it must be destroyed in order to protect the recipient.[42]
Back to beginning
