| Silver nitrate method | [German version] |
Application
When goods are transported by sea, parts of the cargo frequently suffer wetting damage. This damage may be caused by the dampening effect of sweat (condensation) or by the penetration of seawater into the hold or container.
To distinguish between these causes or to rule out of the possibility of seawater damage, a seawater test is performed using the silver nitrate method, which detects the presence of chloride ions.
Figure 1 shows a test bag, which, given its discoloration, has obviously been damaged by water. It is not clear to the eye whether the damage is caused by sweat or seawater.
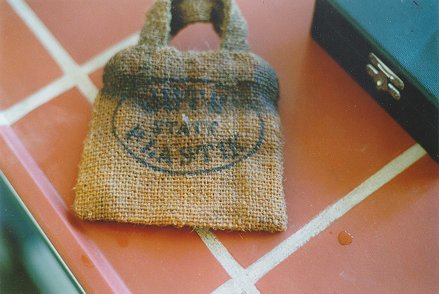 |
| Figure 1 |
The basis of the test is that a liquid containing chloride ions changes immediately into a whitish solution when silver nitrate solution is added (0.1 mol/L). If these chloride ions are not present, the solution remains clear and transparent.
Figure 2 shows the equipment required for the test:
a small bottle, if possible a dropping bottle, of silver nitrate (AgNO3) – protect solution from air; a bottle of distilled water (H2O) and two beakers.
 |
Figure 2 |
At least two samples must always be taken, i.e. one sample from the damaged product and one blank sample, or counter sample, from an area in which no seawater damage can possibly have occurred. Where damage is relatively slight, blank samples may be taken from the underside of the relevant bag or other remote items of cargo which cannot have been damaged.
Blank samples are essential, as it must be established whether chloride ions were already contained in the product or the bags which have not been damaged. If this is the case, the blank sample will also discolor to a greater or lesser degree, meaning that more extensive, laboratory-based tests will then have to be performed. In such an instance, the inspector takes several samples, which are packaged individually in plastic bags and labeled clearly. A plan should be drawn up of the area from which the samples have been taken.
It is best if the presence of seawater can be detected directly on board by the inspector, who can ensure that the samples are taken from areas where seawater penetration is possible.
Test procedure
1. Testing of discolored jute bags for seawater dampening. Take some fibers from the bag allegedly damaged by seawater and put them into glass beaker A, see Figure 3.
2. Proceed in the same way for an undamaged bag located in an area where seawater damage is unlikely. Put this sample in glass beaker B, see Figure 3.
 |
| Figure 3: On the left a sample from the damaged bag, on the right a sample from an undamaged bag. |
3. As a next step, add approximately 100 ml of distilled water to each glass beaker. You may judge the quantity by eye, but take care to introduce as far as possible the same amount into each glass beaker, see Figures 4 and 5.
 |
 |
| Figure 4 | Figure 5 |
4. Now stir both samples in the water and extract any chloride ions present by mechanical agitation and shaking, see Figure 6.
 |
Figure 6 |
5. Then introduce some drops of silver nitrate solution into sample A using a pipette or simply from your dropping bottle (Figures 7 and 8). If the result is positive, the sample will discolor as soon as the first drop has been added.
 |
 |
| Figure 7: Addition of a few drops of silver nitrate solution to sample A | Figure 8: Sample A discolors as soon as the first drop has been added. |
6. Now add silver nitrate to the blank sample. If there is no reaction, and the water remains clear, the first sample A has probably sustained seawater damage, see Figure 9. However, if the color of the second sample also changes, seawater damage is not definite. As described above, several samples are then taken and sent to a laboratory, so that seawater damage may be confirmed or ruled out by more extensive measurements.
 |
Figure 9 |
Figure 10 is a representation of the chemical equation showing precipitation reaction.
 |
| Figure 10 |
Testing of grain or feedstuffs
Figures 11 to 13 show the same test using a grain sample taken from bags which have developed mold as a result of wetting. The blank sample was taken from undamaged bags.
 |
| Figure 11 |
The color change in Figures 12 and 13 shows that salt water and not sweat is the probable cause of the moisture damage which ultimately led to mold growth.
 |
| Figure 12: Color change in sample A after the addition of silver nitrate solution |
 |
| Figure 13: No color change in sample B, from which it may be concluded that the damage is due to seawater. |
Feedstuffs are the most likely cargo to contain chloride ions even when undamaged. More extensive methods may help in this case.
The points from which samples have been taken must be clearly described or photographed.
Unprocessed samples should be secured in sealed plastic bags.
Back to beginning
